Answer:
The pH of the solution is 1.60.
Step-by-step explanation:
1st) It is necessary to write and balance the chemical reaction:

Now we can see that 1 mole of NaOH reacts with 1 mole of HCl.
2nd) We have to calculate the moles contained in 15mL of 0.10M NaOH solution and the moles contained in 25mL of 0.10M HCl solution:
• Moles contained in NaOH solution:
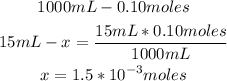
• Moles contained in HCl solution:
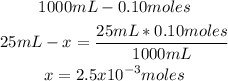
Now we know that there are 1.5x10^-3 moles of NaOH and 2.5x10^-3 moles of HCl.
3rd) According to the stoichiometry of the reaction, 1 mole of NaOH reacts with 1 mole of HCl, so in this case, 1.5x10^-3 moles of NaOH will react with 1.5x10^-3 moles of HCl, because NaOH will be the limiting reactant and HCl will be the excess reactant.
So, now we have to calculate the excess of HCl:
2.5x10^-3moles - 1.5x10^-3moles = 1x10^-3moles
Now we know that there are 1x10^-3 moles of HCl left.
4th) Excess HCl will remain dissociated into H+ and Cl-, according to the following equation:
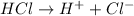
That means that for every mole of HCl, 1H+ dissociates. So, in this case, there are 1x10^-3 moles of H+.
Remember that these moles are contained in 40mL, so the molarity of H+ is 0.025M:
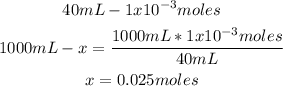
5th) Finally, we can calculate the pH of the solution, by replacing the H+ concentration in the pH formula:
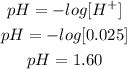
So, the pH of the solution is 1.60.