Answer:
P₄ + 4NaOH + 2H₂O → 2PH₃ + 2Na₂HPO₃
Step-by-step explanation:
The given reaction expression is given as:
P₄ + NaOH + H₂O → PH₃ + Na₂HPO₃
To solve this problem, now we put coefficients a, b, c, d and e and we use a mathematical approach to solve this problem;
aP₄ + bNaOH + cH₂O → dPH₃ + eNa₂HPO₃
Conserving P: 4a = d + e
Na: b = 2e
O: b + c = 3e
H: b + 2c = 3d + e
let b = 1, e =
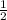 , c =
, c =
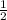 , d =
, d =
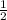 , a =
, a =
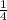
Multiply through by 4;
b = 4, e = 2, c = 2, d = 2, a = 1
P₄ + 4NaOH + 2H₂O → 2PH₃ + 2Na₂HPO₃