Answer: The mole ratio of
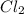 to
to
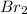 in the given reaction is 1:1 .
in the given reaction is 1:1 .
Step-by-step explanation: For a reaction the mole ratio is the ratio of their coefficients. Coefficients are the numbers used to balance the equations.
The given reaction is:
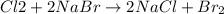
In this equation, the coefficient for each of
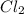 and
and
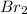 is 1. So, the mole ratio of
is 1. So, the mole ratio of
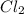 to
to
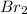 in the given reaction is 1:1 .
in the given reaction is 1:1 .