Answer:
Ka = 3.16 x 10⁻⁸.
Step-by-step explanation:
- We know that: pH = -log[H⁺],
3.75 = -log[H⁺].
log[H⁺] = -3.75.
∴ [H⁺] = 1.78 x 10⁻⁴ M.
- Also, we have that: [H⁺] =
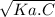
where, Ka is the dissociation constant of the acid,
C is the concentration of the acid (C = 1.0 M).
∴ 1.78 x 10⁻⁴ M = √(Ka)(1.0 M),
by squaring the both sides:
∴ (1.78 x 10⁻⁴ M)² = (Ka)(1.0),
∴ Ka = 3.16 x 10⁻⁸.