Answer : The correct option is, (D) 3 : 1
Explanation :
The balanced chemical equation will be,
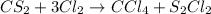
By the stiochiometry, 1 mole of carbon sulfide react with 3 moles of chlorine to give 1 mole of carbon tetrachloride and 2 moles of sulfur chloride.
From the balanced chemical reaction, we conclude that the mole ratio of
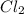 to
to
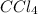 is, 3 : 1
is, 3 : 1
Hence, the correct option is, (D) 3 : 1