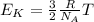Answer: The volume occupied by the air inside the balloon and pressure exerted by the air particles on the walls of ballon will get increased.
Step-by-step explanation:
The temperature of the hot water is higher than the room temperature.
When the balloon with fixed amount of air kept in the hot water. The volume occupied by the air inside the balloon will increase because the temperature of the hot water is more than that of temperature of room temperature.
This is because the volume occupied by the gas is directly proportional to the temperature at constant pressure (Charles law).
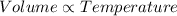
The pressure on the balloon from the external source will remain unchanged but the pressure exerted by the air particles on the walls of balloon will get increased as the volume of the balloon is increasing.
The average kinetic energy of the gas molecules will get increased in hot water as kinetic energy of the gas molecule increases with increase in temperature and it is given by: