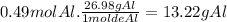Answer:
Step-by-step explanation:
First we have to find the molar mass of the compound.
We must multiply the atomic mass of each element of the compound by the number of atoms of each one
Aluminum oxide:
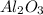
molar mass
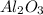
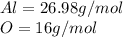
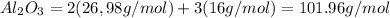
We know that in 1 mol of
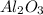 it has a mass of 101.96g.
it has a mass of 101.96g.
Now we can find out how many moles of
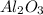 are in 25 g.
are in 25 g.
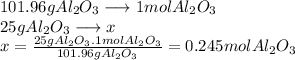
For every mole of
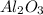 we have 2 moles of Al.
we have 2 moles of Al.
To find out how many moles of Al we have in
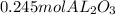 we use a rule of three
we use a rule of three
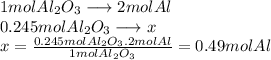
There are 0.49 moles of Al in 25 g of
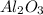
now we calculate the grams of Al in 0.49 moles of Al