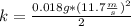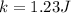Answer:
Step-by-step explanation:
1. The equation to calculate the kinetic energy is:
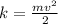 (Eq.1)
(Eq.1)
where, m=mass, v=velocity, k=kinetic energy
2. Find the mass of one mole of water,
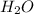 :
:
Atomic weight of H = 1
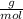
Atomic weight of O = 16
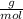
Molecular weight of
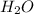 = (2*1) + 16
= (2*1) + 16
Molecular weight of
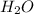 = 18
= 18
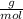
3. Convert the mass of
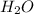 from g to Kg:
from g to Kg:
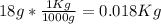
3. Replace quantities in Eq.1: