The pressure is doubled(2 atm)
Further explanation
Given
50 L of CO₂ gas at STP
Required
The pressure
Solution
Changing pressure, constant temperature⇒Boyle's Law
At a fixed temperature, the gas volume is inversely proportional to the pressure applied
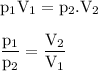
STP⇒1 atm, T=273 K
The (new) volume of gas in half, so V₂=0.5 V₁ = 0.5 x 50 = 25 L
Because the gas volume is inversely proportional to the pressure than the pressure doubled = 2 atm
Or we can use the formula :
P₂=(P₁V₁)/V₂
P₂=(1 atm x 50 L)/25 L
P₂= 2 atm