Hello!
Find the relative rate of diffusion for the gases chlorine, CL2 and ethane, C2H6
- We have the following data:
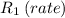
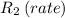
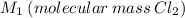
Cl2 = 2*(35.5u) = 71u → M1 = 71 g/mol
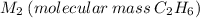
C2 = 2*(12u) = 24u
H6 = 6*(1u) = 6u
C2H2 = 24u + 6u = 30u → M2 = 30 g/mol
*** Note: Under Graham's Law, the rate of diffusion and effusion of gases (volume that leaks per unit time, that is, in seconds) is inversely proportional to the square root of your molar masses.
- We apply the data to Graham's formula (Law of Effusion and Diffusion of gases):
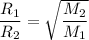


Answer:
The relative rate of diffusion for the gases chlorine and ethane is 65%
________________________