The new temperature (T₂) in the lab = 25.09 °C
Further explanation
Given
V₁=2.15 ml
T₁ = 17 + 273 = 290 K
P = 740.2 mmHg
V₂ = 2.21 ml
Required
The new temperature (T₂)
Solution
Charles's Law
When the gas pressure is kept constant, the gas volume is proportional to the temperature
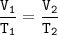
Input the value :
T₂=(T₁.V₂)/V₁
T₂=(290 K x 2.21 ml)/2.15 ml
T₂=298.09 K = 25.09 °C