Step-by-step explanation:
Density is defined as mass divided by volume.
Mathematically, Density =
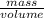
It is given that mass is 35.4 g and volume is 36.82 ml. Since there are 1000 ml in 1 L so, 36.80 ml will be equal to 0.03682 L.
Hence, calculate the density as follows.
Density =
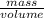
=
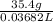
= 961.43 g/L
Thus, we can conclude that density of the given sample is 961.43 g/L.