Answer:
See the explanation.
Step-by-step explanation:
Hello,
In this case, the overall double displacement undergoing chemical reaction turn out into:
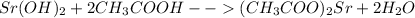
The names are:
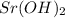 : strontium hydroxide.
: strontium hydroxide.
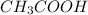 : acetic acid.
: acetic acid.
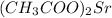 : strontium acetate.
: strontium acetate.
 : water.
: water.
Now, the net ionic equation is obtained from the following procedure:
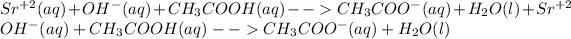
In which it is seen that the strontium ion is cancelled as it is completely dissociated unlike the acetate at the left side.
Best regards.