Answer:
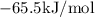
Explanations:
Given the hypothetical thermochemical equation, expressed as:
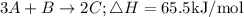
We are to find the enthalpy change (ΔH ) for the reaction 2C → 3 A + B.
• You can see that the, given reaction, 2C → 3 A + B is the, reverse ,of the thermochemical equation 3 A + B → 2 C.
• Since the ,reverse reaction is possible,, the reaction enthalpy has the ,same numerical value, but with the, ,opposite sign
Therefore, the enthalpy change ∆H, in kJ/mol, be for the reaction 2 C → 3 A + B is -65.5kJ/mol