Answer: The mole ratio of Zn to ZnO is 3: 3.
Step-by-step explanation:
According to law of conservation of mass, mass can be neither be created nor be destroyed. The mass on reactant side must be equal to the mass on product side. Thus the atoms of each element on both side of the reaction must be same.
The balanced chemical reaction is:
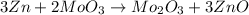
Here 3 moles of Zn combine with 2 moles of
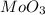 to give 1 mole of
to give 1 mole of
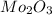 and 3 moles of
and 3 moles of
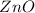 . Thus the mole ratio of Zn to ZnO is 3: 3.
. Thus the mole ratio of Zn to ZnO is 3: 3.