Answer: Number of neurons in
Helium = 1
Carbon = 8
Nitrogen = 8
Strontium = 52
Tellurium = 71
Step-by-step explanation: Isotopes are the forms of same element having different mass numbers. They only differ in the number of neutrons. The number of protons remain same.
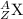 and
and
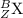 are the two isotopes of same element X.
are the two isotopes of same element X.
Neutrons can be calculated by:
Number of neutrons = Mass Number - Number of neutrons
and Atomic Number = Number of protons
For the given elements, Mass numbers are given
Atomic Number of Helium = 2
Given mass number = 3
Number of neutrons in Helium= (3 - 2) = 1
Similarly,
Number of neutrons in Carbon = (14 - 6) = 8
Number of neutrons in Nitrogen = (15 -7) = 8
Number of neutrons in Strontium = (90 - 38) = 52
Number of neutrons in Tellurium = (123 - 52) = 71