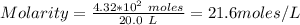Answer:
Molarity of the solution = 21.6 M
Step-by-step explanation:
Given:
Moles of CH#COOH = 4.32*10^2 moles
Volume of water = 20.0 L
To determine:
Molarity of the solution
Step-by-step explanation:
Concentration of a given solution can be expressed in terms of Molarity which refers to the moles of solute per liter volume of the solution
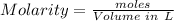
For the given CH3COOH solution: