Answer: The correct answer is Option A.
Step-by-step explanation:
Titanium is the 22nd element of the periodic table with electronic configuration of
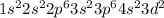
In the naming of the compound, the oxidation state of this element is given +3.
Oxygen is the 8th element of the periodic table with electronic configuration
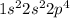
This element will easily gain 2 electrons and shows an oxidation state of -2.
By criss-cross method, the oxidation state of titanium and oxygen gets reversed and they form the coefficients of the compound.
The compound formed by the combination of Titanium(III) and oxygen is
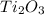 having chemical name of Titanium (III) oxide.
having chemical name of Titanium (III) oxide.
Thus, the correct answer is Option A.