Answer:
1.44moles
Step-by-step explanation:
Given parameters:
Number of atoms = 8.86 x 10²³atoms
Unknown:
Number of moles = ?
Solution:
From the mole concept;
Number of moles =
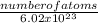
Number of atoms = 8.86 x 10²³atoms
Number of moles =
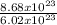
Number of moles = 1.44moles