Answer: the best option to answer the question is the second one (or letter B), "2"
Step-by-step explanation:
The question requires us to identify how many electrons a calcium (Ca) atom would lose to form an ion.
We can analyze the electron configuration of Ca to understand how many electrons it would lose.
The atomic number of Ca is 20, thus its electron configuration is as it follows:
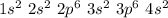
From the electron configuration, we can see that Ca presents 2 electrons in its valence shell (4s2). To achieve stability, it is easier for Ca to lose these two electrons and form a Ca2+ ion.
Therefore, the best option to answer the question is the second one (or letter B), "2".