The correct answer is option Covalent bonds.
The water is a molecule formed by the covalent interaction between one oxygen and two hydrogen molecule. The chemical formula of the water is
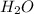 . In the water molecule, each hydrogen atom shares an electron with the oxygen. The oxygen is more electronegative so, it pulls the electron towards it, and gains a partial negative charge and the hydrogen gains a partial positive charge. The partial positive charge of hydrogen in one molecule attracts the partial negative charge in other molecule and forms inter molecule hydrogen bonding.
. In the water molecule, each hydrogen atom shares an electron with the oxygen. The oxygen is more electronegative so, it pulls the electron towards it, and gains a partial negative charge and the hydrogen gains a partial positive charge. The partial positive charge of hydrogen in one molecule attracts the partial negative charge in other molecule and forms inter molecule hydrogen bonding.