Answer
V₂ = 1.9 L
Step-by-step explanation
Given:
P₁ = 1.5 atm
V₁ = 3.0 L
T₁ = 20⁰C = (20 + 273 )K = 293 K
P₂ = 2.5 atm
T₂ = 30⁰C = (30 + 273 )K = 303 K
What to find:
V₂
Step-by-step solution:
The unknown V₂ can be determined using the combined gas equation, which is given below.
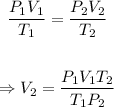
Putting the values of the given parameters into the equation, we have
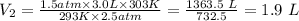
Therefore, the unknown V₂ in number 1 is 1.9 L