Answer:
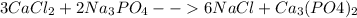
Step-by-step explanation:
Hello,
In this case, the required balanced chemical reaction is:
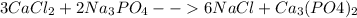
At first, 3 calciums are needed at the reactants to balance it, therefore, 2 phosphates are needed at the reactants as well, for that reason, a six is needed at the sodium chloride to balance chlorine and consequently sodium
Best regards.