Answer: These two differ by the number of electrons.
Step-by-step explanation:
Atom is defined as the smallest unit of matter which cannot be further divided. A neutral atom has same number of protons and electrons present in it. This determines the atomic number of that atom.
An ion is formed when an atom looses or gains electron.
- When an atom looses electrons, it will form a positive ion known as cation.
- When an atom gains electrons, it will form a negative ion known as anion.
Thus, an ion has unequal number of protons and electrons.
For Example: Taking Sodium element having atomic number 11
Number of electrons in neutral atom = 11
Number of protons in neutral atom = 11
This atom forms a positive ion,
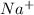
Number of electrons in ion = 10
Number of protons in ion = 11
Hence, an atom and an ion of the same element has different number of electrons.