Well first you would have to figure out the raw amount of solution you wanted to make. Then you would have to rearrange the molarity formula to calculate the number of moles you would need of sodium bicarbonate to make your amount solvent into a 5 molar solution. The formula you would need to calculate moles needed would be
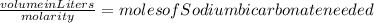
. Then take the molar mass of Sodium Bicarbonate by adding up all of the elements weights and times it by the amount of moles needed to get the amount of grams needed to make your 5 molarity solution.