Answer:
n = 0.36 mol
Step-by-step explanation:
We need to find number of moles present in 16 grams of carbon dioxide.
Let there are n number pf moles,
n = given mass/molecular mass
The molecular mass of carbon dioxide = 44 g/mol
So,
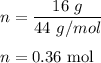
So, there are 0.36 moles in 16 grams of carbon dioxide.