Answer:
The work is 9.99J.
Step-by-step explanation:
The work associated with a process is given by the following formula:
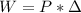
In which W is the work, P is the pressure and
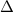 is the difference of volume.
is the difference of volume.
In this problem, we have that:
The volume of an ideal gas is decreased from 5.0 l to 5.0 ml. Each ml has 0.001l. So the volume of the ideal gas is decreased from 5.0l to 0.005l. So
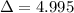 .
.
Constant pressure of 2.0 atm. So
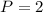 .
.
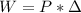
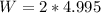
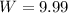
The work is 9.99J.