Answer:
100 million hydrogen atoms.
Step-by-step explanation:
To find the number of hydrogen atoms lined side by side that would make the line 1 cm long, we divide the total length i.e 1 cm by the size of each hydrogen atom i.e 0.1 nm.
First we convert all given values to meter
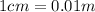
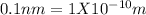
Now we can divide the converted values
No of hydrogen atoms =
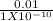
= 100,000,000 =
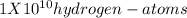
i.e 100 million hydrogen atoms of diameter 0.1 nm would be lined side by side to make a line 1 cm long.