Answer: A substance with a pH of 2 has a 10 times higher concentration of H+ than a substance with a pH of 3.
Explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration and pOH is calculated by taking negative logarithm of hydroxide ion concentration.
![pH=-\log [H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/y1nlg9qxar6fauop1r05a1g4xt6dhnvirc.png)
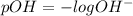
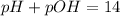
Given: pH=2
![2=-log[H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/7ywv6rlwjtdpqsy3lra28attpoyw9hyueo.png)
![[H^+]=10^(-2)M](https://img.qammunity.org/2018/formulas/chemistry/high-school/y5ozpd24te6x8tj5vvbbu44a0nmispzs24.png)
pH=3
![3=-log[H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/odhvil127f97ra7obh18u2naqgx3qu5xds.png)
![[H^+]=10^(-3)M](https://img.qammunity.org/2018/formulas/chemistry/high-school/cfj72ldogonz5ow5fqxqqqd16r48ghgklj.png)
A substance with a pH of 2 has a 10 times higher concentration of H+ than a substance with a pH of 3.