Complete Question:
When a 40.0-g nugget of pure gold is heated from 49°C to 60.0°C, it absorbed 5339.01 of energy. What is
the specific heat of gold?
Answer:
Specific heat capacity, c = 12.134J/KgC
Step-by-step explanation:
Given the following data;
Mass = 40g
Initial temperature, T1 = 49°C
Final temperature, T2 = 60.0°C
Quantity of heat = 5339.01J
To find the specific heat capacity of gold;
Heat capacity is given by the formula;
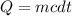
Where;
- Q represents the heat capacity or quantity of heat.
- m represents the mass of an object.
- c represents the specific heat capacity of water.
- dt represents the change in temperature.
dt = T2 - T1
dt = 60 - 49
dt = 11°C
Substituting the values into the equation, we have;
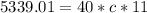
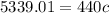
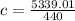
Specific heat capacity, c = 12.134J/KgC