The mass of 63 ml sample : 79.38 g
Further explanation
Given
20 ml and 25.2 g of glycerol
Required
The mass of 63 ml sample
Solution
Density is the ratio of mass per unit volume
Density formula:
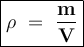
Density of glycerol :
= m : V
= 25.2 g : 20 ml
= 1.26 g/ml
Mass of 63 ml sample :
= density x volume
= 1.26 g/ml x 63 ml
= 79.38 g