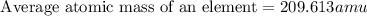Answer: B. Relative abundance of each isotope
Step-by-step explanation:
Average atomic mass is the average mass of all the isotopes present depending on the relative abundance of each isotope.
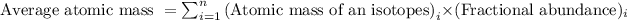
Example:
Mass of isotope 1 = 203.973 amu
relative abundance of isotope 1 = 0.014
Mass of isotope 2 = 205.9745 amu
relative abundance of isotope 2= 0.241
Mass of isotope 3 = 206.9745 amu
relative abundance of isotope 3 = 0.221
Mass of isotope 4 = 207.9766 amu
relative abundance of isotope 4 = 0.574
Formula used for average atomic mass of an element :
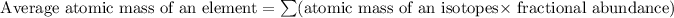
![\text{ Average atomic mass of an element}=\sum[(203.973*0.014)+(205.9745* 0.241)+(206.9745* 0.221)+(207.9766* 0.574)]](https://img.qammunity.org/2018/formulas/chemistry/high-school/l02a0acfmoxxw48z30fknsrs4njh9dcqpq.png)