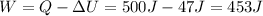We can solve the problem by using the first law of thermodynamics:
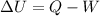
where
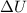 is the variation of internal energy of the system
is the variation of internal energy of the system
Q is the heat added to the system
W is the work done by the system
In this problem, the variation of internal energy of the system is
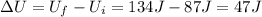
While the heat added to the system is

therefore, the work done by the system is