Answer: The rate of disappearance ( -Δ[B]/Δt) under the same conditions is 0.200 M/s.
Step-by-step explanation:
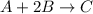
Initial Rate of the reaction = 0.100 M/s
Rate of disappearance of B:
![-(1)/(2)(\Delta [B])/(\Delta t)=0.100 M/s](https://img.qammunity.org/2018/formulas/chemistry/college/pbdt88rsulmullzzpanwozzmh7k0jwzpis.png)
![-(\Delta [B])/(\Delta t)=2* 0.100 M/s=0.200 M/s](https://img.qammunity.org/2018/formulas/chemistry/college/73su4refvp7o3ad1vyx1qycnftez5nj990.png)
The rate of disappearance ( -Δ[B]/Δt) under the same conditions is 0.200 M/s.