Answer:
291.72 grams of magnesium metal will react completely with 4.8 liters of 5.0 M HCl.
Step-by-step explanation:
Molarity of teh HCl solution = 5.0 M
Volume of HCl solution = 4.8 L

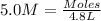
Moles of HCl = 24 moles
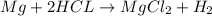
According to reaction , 2 moles of HCl reacts with 1 mole magnesium.
Then 24 moles of HCl will react with:
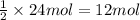 of magnesium
of magnesium
Mass of 12 mol of magnesium =24.31 g/mol × 12 mol = 291.72 g
291.72 grams of magnesium metal will react completely with 4.8 liters of 5.0 M HCl.