Answer: The work done by the system is 800 Joules.
Step-by-step explanation:
Internal change in thermal energy of the system,
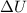 = 600 Joules
= 600 Joules
Energy added to the system the system,Q = 1400 Joules
Work done by the system can be calculated by using First law of thermodynamics:'
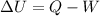 (First law of thermodynamics)
(First law of thermodynamics)
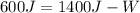
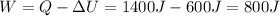
The work done by the system is 800 Joules.