Answer:
D. atomic masses
Step-by-step explanation:
Molecular formulas is the actual number of atoms of each element in the compound. Molecular mass is the sum of the mass of each element which is present in the molecular formula is multiplied by their subscript which appear in the molecular formula.
For example,
The formula is =
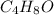
Mass from the formula = 4×Mass of Carbon + Mass of Hydrogen×1 + Mass of oxygen = 4×12 + 8×1 + 16= 72 g/mol
Thus, Correct option is - D. atomic masses