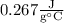Answer: Approximately
===================================================
Work Shown:
We have the following variables
- Q = 714 joules = heat required
- m = 52 grams = mass
- c = specific heat = unknown
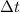 = 82-30.5 = 51.5 = change in temperature
= 82-30.5 = 51.5 = change in temperature
note: the symbol
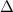 is the uppercase Greek letter delta. It represents the difference or change in a value.
is the uppercase Greek letter delta. It represents the difference or change in a value.
Apply those values into the formula below. Solve for c.
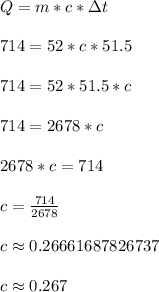
The specific heat of the unknown metal is roughly