Given:
The initial amount of substance, No=10 g.
The amount of substance left after 9 years, N=5 g.
Since 10 g of substance is present initially, and it became 5 g(half of the initial amount) in 9 years, the half life of the substance is, t =9 years.
Hence, the expression for the amount remaining after T years is,
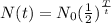
To find the amount of substance remaining after 18 years, put T=18, N0=10 and t=9 in the above equation.
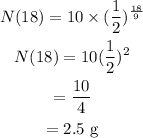
Therefore, after 18 years 2.5 g of the radioactive substance will remain.