The molarity of a Sodium carbonate solution : 0.373 M
Further explanation
Given
32.52 g Na₂CO₃
822 ml of solution = 0.822 L
Required
The molarity
Solution
Molarity shows the number of moles of solute in every 1 liter of solution.
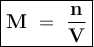
mol of solute = mol of Na₂CO₃ :
= mass : MW Na₂CO₃
= 32.52 g : 106 g/mol
= 0.307
Molarity :
= n : V
= 0.307 mol : 0.822 L
= 0.373 M