Answer:
a. 25.5 moles of Pb will be formed.
b. 54.8 moles of PbO are needed.
c. PbS is the limiting reactant.
Step-by-step explanation:
1st) It is necessary to write the balanced chemical reaction:
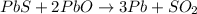
From the balanced chemical reaction we know that 1 mole of PbS reacts with 2 moles of PbO to produce three moles of Pb and 1 mole of SO2.
2nd) Using the stoichiometry of the reaction and a mathematical rule of three, we can calculate the moles of Pb that will be produced from 17 moles of PbO and excess of PbS:
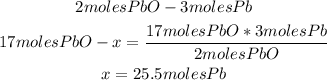
So, 25.5 moles of Pb will be formed.
3rd) Using the stoichiometry of the reaction and a mathematical rule of three, we can calculate the moles of PbO that are needed to make 27.4 moles of SO2 in excess of PbS:
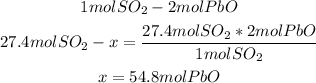
So, 54.8 moles of PbO are needed.
4th) Using the stoichiometry of the reaction we can calculate the limiting reactant:
- Calculation from 17.6 moles of PbS:
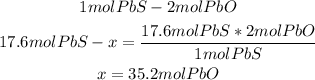
From the stoichiometry of the reaction 1 mol of PbS reacts with 2 moles of PbO, so the 17.6 moles of PbS will need 35.2 moles of PbO to react properly, but we have 36g of PbO, so PbO will be the excess reactant and PbS the limiting reactant.
- Calculation from 36 moles of PbO:
We can do this calculation to confirm the previous one:
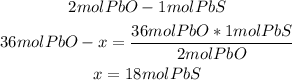
In this case, we can see that 36 moles of PbO will need 18 moles of PbS to react properly, but we only have 17.6 moles of PbS. Here we confirm that PbS is the limiting reactant.