Answer : The correct option is
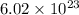 .
.
Explanation :
One mole of substance means the amount of substance that contains as many particles in pure substance. So, one mole contains
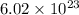 of particles. And this is also known as Avogadro's number.
of particles. And this is also known as Avogadro's number.
Therefore, the
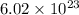 is value which gives the number of particles in one mole of a substance.
is value which gives the number of particles in one mole of a substance.