The volume of concentrated HCl : 2.073 ml
Further explanation
Given
37% HCl by mass; density 1.19 g/mL
Required
The volume of concentrated HCl
Solution
Conversion to molarity :
37% x 1.19 g/ml =0.4403 g/ml
g/ml to mol/L :
=0.4403 g/ml x 1000 ml/L : 36.5 g/mol
=12.06 mol/L
or we can use formula :
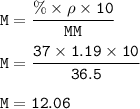
Dilution formula :
M₁V₁=M₂V₂
12.06 M x V₁ = 0.1 M x 0.25 L
V₁ = 0.0021 L = 2.073 ml