Answer:
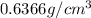 is the density of the cubic plastic.
is the density of the cubic plastic.
Step-by-step explanation:
Mass of the cubic block= m = 1.1 g
Volume of the cubic block = V
Volume of the cube =
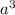
a = side length =
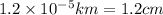
1 km = 100,000 cm
Volume of cubic block =
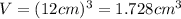
Density of the solid block,d = ?
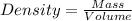
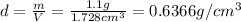
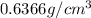 is the density of the cubic plastic.
is the density of the cubic plastic.