Answer:The boiling point of the water by adding 2.5 moles of aluminium nitrate will be changed by
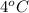 .
.
Step-by-step explanation:
When sugar is dissolved is x kilograms of water
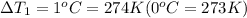
Number of moles of sugar dissolved = 2.5 mol
i = Van'T Hoff factor of sugar = 1
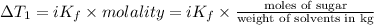
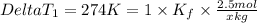 ..(1)
..(1)
When aluminium nitrate is dissolved x kilogram of water:
Number of moles of aluminium nitrate dissolved = 2.5 mol
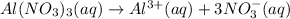
i' = Van'T Hoff factor of aluminium nitrate = 4
Aluminium nitrate being ionic compound will get dissociated into its constituting ions. The value of ' i' ' of the ionic compound is equal to number of discrete ions in a formula unit of a substance.
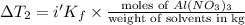
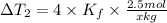 ...(2)
...(2)
On dividing (1)and (2), we get
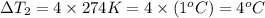
The boiling point of the water by adding 2.5 moles of aluminium nitrate will be changed by
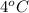 .
.