Answer:
3.01×10²³molecules.
Explanations:
The formula for the number of molecules of a compound given the number of moles is expressed as:
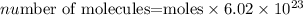
Get the moles of ethane gas using the formula:

Determine the required number of molecules of ethane
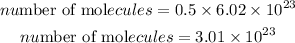
Hence the molecule of ethane gas that is in 15 grams of the compound is 3.01×10²³molecules.