Answer: 145.87 grams of ammonia will produce the given amount of nitrogen.
Step-by-step explanation:
To calculate the moles of nitrogen, we use the formula:
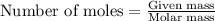 ....(1)
....(1)
Given mass of nitrogen = 300 g
Molar mass of nitrogen = 28.02 g/mol
Putting values in above equation, we get:
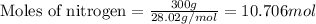
For the given reaction:
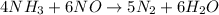
By Stoichiometry of the reaction:
5 moles of nitrogen is produced from 4 moles of ammonia.
So, 10.706 moles of nitrogen will be produced from =
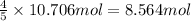
Now, calculating the mass of ammonia, we use equation 1:
Molar mass of ammonia = 17.0337 g/mol
Moles of ammonia = 8.564 mol
Putting values in equation 1, we get:
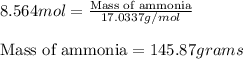
Hence, 145.87 grams of ammonia will produce the given amount of nitrogen