Answer:
2.0724moles
Explanations:
Given the balanced chemical reaction between Na2CO3 and CaCl2 expressed as:
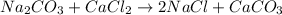
Given the following parameters
Mass of CaCl₂ = 115g
Determine the moles of CaCl₂
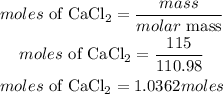
According to stochiometry, 1 mole of CaCl2 prodcues 2 moles of NaCl. The moles of NaCl requred is given as:
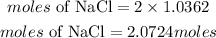
Therefore moles of NaCl made is 2.0724moles