Answer : The number of oxygen molecules that do not engage in the reaction are 50 molecules.
Explanation :
The balanced chemical reaction is,
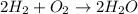
From the balanced chemical reaction we conclude that,
As, 2 molecules of hydrogen react with 1 molecule of oxygen
So, 300 molecules of hydrogen react with
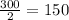 molecules of oxygen
molecules of oxygen
The number of oxygen molecules reacted = 150 molecules
Now we have to determine the number of oxygen molecules that do not engage in the reaction.
Number of oxygen molecules left = Given number of oxygen molecules - Number of oxygen molecules reacted
Number of oxygen molecules left = 200 - 150
Number of oxygen molecules left = 50 molecules
Therefore, the number of oxygen molecules that do not engage in the reaction are 50 molecules.