Answer:
The correct answer is
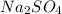 .
.
Step-by-step explanation:
Given polyatomic ions:
1) Sulfate =
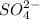
2) Phosphate =
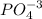
3) Hydroxide =
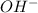
The formula of Sodium sulfate will be:
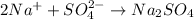
Sodium carries 1+ charge and and sulfate ion carries 2-charge. In order to neutralize negative charge on sulfate ion two sodium ions will be needed.